Graseby(MDKMed) Medical focuses on the two major fields of acute and critical care and minimally invasive surgery. Relying on its strong R&D abilities, the company integrates the world’s cutting-edge technical concepts, deeply explores user needs and provides global customers with medical products including vital sign monitoring, drug infusion, respiratory therapy, endoscopy system, acute and critical clinical decision support system.
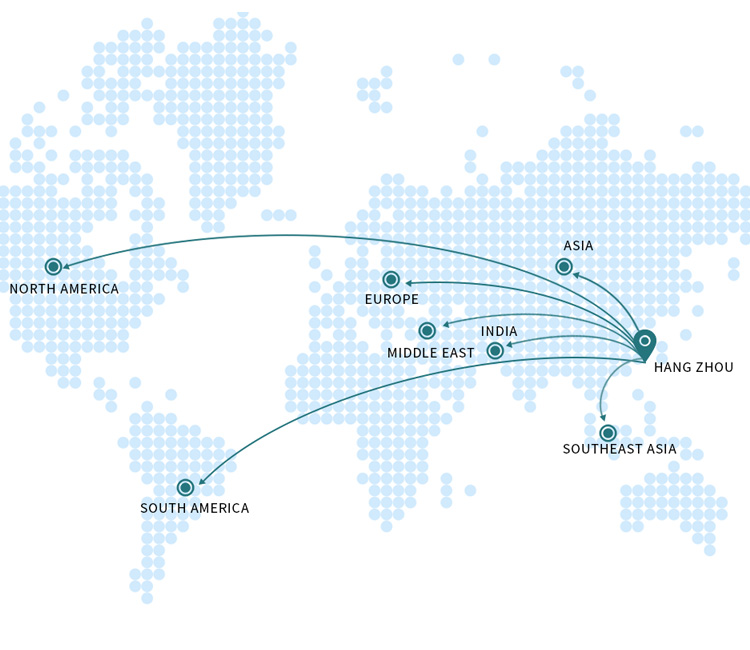
Graseby(MDKMed) Medical has established modern production bases in Hangzhou and Lishui in Zhejiang province, which conform to the quality management system of medical production. Graseby(MDKMed) Medical has successively set up overseas offices in Germany, the United States, the Middle East, South Asia, South America, Southeast Asia to comprehensively accelerate its global strategic plan. Graseby(MDKMed) Medical has quickly become the supplier with the most complete product line in acute and critical care and minimally invasive surgery for hospitals all over the world. With its slogan ‘Winning at interconnection’, Graseby(MDKMed) Medical is providing more space of imagination for global healthcare.

R&D team: The R&D team is led by national distinguished experts and is composed of senior engineers and experts in the industry. Most of the R&D personnel come from prestigious universities at home and abroad, and 20% of them have doctoral and master degrees from internationally renowned universities.
Qualification patents: The company’s quality system has passed ISO13485 and other medical device quality control system certifications, and has relevant medical device production qualifications and sales licenses. Products on sale have obtained EU CE (European Conformity) or CFDA certificates. The products have more than 60 independent intellectual property patents, and won awards in the National Entrepreneurship and Innovation Competition.

Hangzhou R & D Center

Beijing R & D Center

Graseby(MDKMed) Medical has established modern production bases in Hangzhou, Lishui and Haining in Zhejiang province, which conform to the quality management system of medical production.The production of pump products only, can reach more than 100,000 units per year, and the gross annual value of the consumable workshop that will be put into production is 50 million.


Graseby(MDKMed) has established a complete and scientific medical device quality control system, to ensure that every link from material selection, design and development, test review, prototype trial production, etc. has strict control standards and traceability. Under the strict production management and quality control, ensuring material quality and product performance are 100% reach the requirement. Also, the products are reliable and safe for clinical usage in different environments.
Currently, all the products on sale of Graseby(MDKMed) have relevant medical device production qualifications and sales licenses. The company’s quality system and a variety of products have passed ISO13485 medical device quality control system certification, European Union CE certification (European Conformity), and China CFDA certification.
Brazil
Argentina
Germany
Holland
Middle East
India
Beijing,China
Hangzhou, China
Being global is Graseby(MDKMed) main strategy. Providing customers with high-quality, cost-effective and reliable medical equipment. At present, it has cooperated deeply with many domestic high-level hospitals and high-standard authorized distributors to establish a complete sales and service network. While continuously consolidating the domestic market, it vigorously expands overseas markets. Up to now, it has established a number of branches overseas to support the rapid development of the company’s overseas business. Products are exported to Brazil, Argentina, the Middle East, India, Germany, the Netherlands, Belgium and other countries and regions, which are favored and recognized by overseas customers.
为什么选择我们-13-英文-13-scaled.jpg)


